Technology in a nutshell
Immunotherapeutic strategies to recruit T cells against tumor cells, i.e. checkpoint inhibitors (CPIs), bispecific antibodies (bsAbs) as well as the functionally closely related CART cells have become a cornerstone of oncological treatment. However, so far durable therapeutic success is limited to a minority of patients in case of CPIs, and. in case of bsAbs and CART cells, almost exclusively to hematopoietic malignancies (rather than solid tumors). These shortcomings are due to three major limitations:
- insufficient accessibility of tumor site for immune effector cells in case of solid tumors (in contrast to hematopoietic neoplasias)
- dose - and thus efficacy - limiting side effects due to toxicity, due to lack of truly tumor-specific target antigens (TAAs)
- particularly in case of bsAbs, lacking costimulation resulting in not sufficiently sustained T cell activation
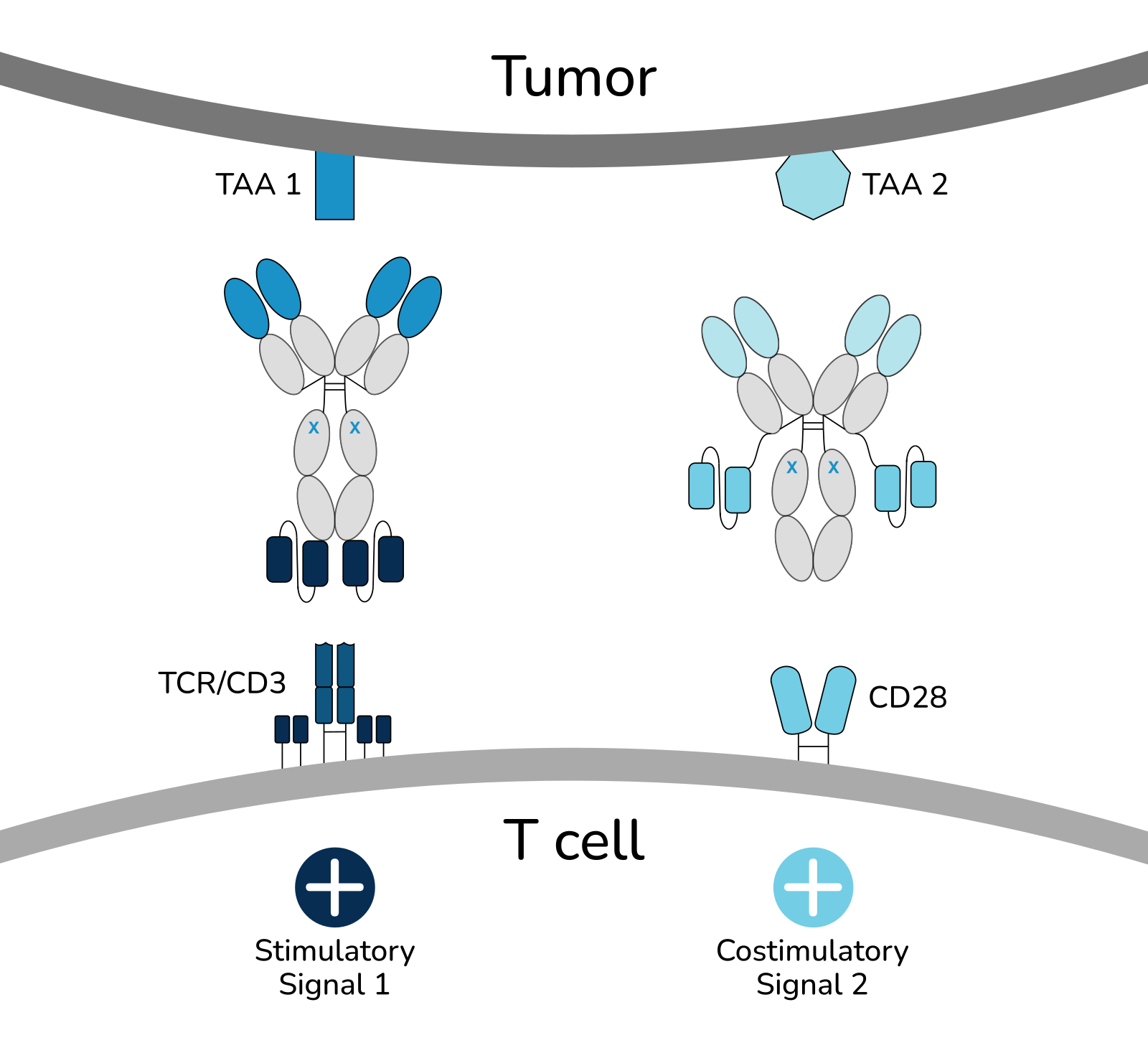
In general, bsAbs appear conceptually ideal, among the three T cell-based therapeutic approaches clinically available to date, as they allow to specifically direct T cells against tumor cells (in contrast to CPIs) and are ready to use “off the shelf reagents” not requiring time-consuming and costly preparative work (in contrast to CART cells). However, almost all bsAbs presently in development comprise anti-CD3 as effector part (bsAbCD3) and thus do not provide for costimulation, resulting in suboptimal T cell activation resulting in exhaustion.
Within TWYCE, we develop and clinically validate a new proprietary class of reagents termed bispecific costimulators (BiCos) with TAAxCD28-specificity, which in combination with specifically selected bsAbs directed to CD3 overcome all the above stated 3 limitations and enable superior T cell immunotherapy of cancer:

Specificity
Targeting a combination of tumor-associated antigens (TAA) that is not found/expressed together on healthy tissues

Accessibility
Targeting antigens expressed on both, tumor cells and microenvironment improves influx of T cells into tumor site

Sustainability
Pronounced and durable T cell immune response by providing two activating signals via CD3 and CD28
Two Targets + Two Signals for safe and truly effective immunotherapy of solid tumors
Pipeline
|
|
|
|
|
|
|
|---|---|---|---|---|---|
| PSMAxCD3 (CC-1) mono in CRPC¹ i.v. | Completed | ||||
|
|
|
|
|
|
|
| CC-1 mono for BCR-PC² | Q2/26 | ||||
|
|
|
|
|
|
|
| CC-1 mono in CRPC¹ s.c. | Q2/26 | ||||
|
|
|
|
|
|
|
| CD276xCD3 (CC-3) mono (basket trial)³ | Q2/26 | ||||
|
|
|
|
|
|
|
| CC-1 + multipeptide vaccine⁴ in CRPC¹ | Q2/25 - Q4/26 | ||||
|
|
|
|
|
|
|
| FIH basket trial ENGxCD28 (BiCo-1)⁵ | 2025 – 2027 | ||||
|
|
|
|
|
|
|
| CC-3 + BiCo-3 (basket trial)³ | 2026 – 2028 | ||||
|
|
|
|
|
|
|
| Preclinical Program |
CC and Bico are proprietary bispecific platforms comprising IgG-based molecules that stimulate CD3 and CD28, respectively, in a strictly target cell-restricted manner.
In the discovery center, several CC and BiCo constructs directed to additional targets as well as yet undisclosed platform technology is being developed.
